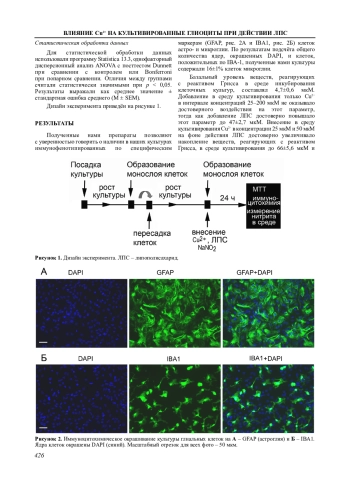
Ионы меди (Cu2+) в концентрации 25–50 мкМ стимулируют вызванную липополисахаридом (ЛПС) продукцию оксида азота (NO) в культурах глиальных клеток, полученных из коры головного мозга крыс и содержащих как астроциты, так и клетки микроглии. Более высокая концентрация Cu2+ (100 мкМ) при стимуляции ЛПС не вызывала достоверного повышения NO в среде инкубации, а при 200 мкМ Cu2+ происходило снижение этого параметра по сравнению с ЛПС. Ионы Cu2+ в этих концентрациях снижали жизнеспособность культивируемых клеток. Видимо, снижение жизнеспособности клеток не связано с накоплением нитритов, так как добавление в среду культивирования даже 100 мкМ нитрита натрия не снижало выживаемость клеток и не влияло на цитотоксичность Cu2+. Исследование клеток микроглии (маркер IBA1) показало, что в культурах, обработанных ЛПС, микроглия имела преимущественно распластанную амебоидную морфологию, характерную для активированной микроглии. Кроме того, под действием ЛПС происходило увеличение площади профильного поля тела клеток и периметра. В концентрации 25 мкМ ионы Cu2+ не влияли на морфологические изменения клеток микроглии, связанные с воспалительным фенотипом. Нельзя исключать, что усиление ионами меди продукции NO, вызванной ЛПС, опосредовано астроцитами.
Идентификаторы и классификаторы
- SCI
- Химия
Нейровоспаление и нарушение гомеостаза меди являются ключевыми патологическими признаками нескольких нейродегенеративных заболеваний, включая болезни Вильсона-Коновалова, Паркинсона и Альцгеймера [1–3]. За последние годы во многих исследованиях было продемонстрировано провоспалительное действие ионов меди [4–6].
Список литературы
1. Wen Y., Zhao C., Chen J., Tian L., Wu B., Xie W., Dong T. (2024) Gandouling regulates ferroptosis and improves neuroinflammation in Wilson’s disease through the LCN2/NLRP3 signaling pathway. J. Inflamm. Res., 17, 5599-5618. DOI: 10.2147/JIR.S465341
2. Zhou Q., Zhang Y., Lu L., Zhang H., Zhao C., Pu Y., Yin L. (2022) Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem. Toxicol., 168, 113369. DOI: 10.1016/j.fct.2022.113369
3. Chen L.L., Fan Y.G., Zhao L.X., Zhang Q., Wang Z.Y. (2023) The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem., 131, 106301. DOI: 10.1016/j.bioorg.2022.106301
4. Aloysius Dhivya M., Sulochana K.N., Bharathi Devi S.R. (2022) High glucose induced inflammation is inhibited by copper chelation via rescuing mitochondrial fusion protein 2 in retinal pigment epithelial cells. Cell. Signal., 92, 110244. DOI: 10.1016/j.cellsig.2022.110244
5. Zhang L., Tsai I.C., Ni Z., Chen B., Zhang S., Cai L., Xu Q. (2024) Copper chelation therapy attenuates periodontitis inflammation through the cuproptosis/autophagy/lysosome axis. Int. J. Mol. Sci., 25(11), 5890. DOI: 10.3390/ijms25115890
6. Guo H., Jing L., Xia C., Zhu Y., Xie Y., Ma X., Fang J., Wang Z., Zuo Z. (2024) Copper promotes LPS-induced inflammation via the NF-κB pathway in bovine macrophages. Biol. Trace Elem. Res., 202(12), 5479-5488. DOI: 10.1007/s12011-024-04107-6
7. Deng H., Zhu S., Yang H., Cui H., Guo H., Deng J., Ren Z., Geng Y., Ouyang P., Xu Z., Deng Y., Zhu Y. (2023) The dysregulation of inflammatory pathways triggered by copper exposure. Biol. Trace Elem. Res., 201(2), 539-548. DOI: 10.1007/s12011-022-03171-0
8. Rossi-George A., Guo C.J., Oakes B.L., Gow A.J. (2012) Copper modulates the phenotypic response of activated BV2 microglia through the release of nitric oxide. Nitric Oxide, 27(4), 201-209. DOI: 10.1016/j.niox.2012.07.002
9. Cuzzocrea S., Persichini T., Dugo L., Colasanti M., Musci G. (2003) Copper induces type II nitric oxide synthase in vivo. Free Radic. Biol. Med., 34(10), 1253-1262. DOI: 10.1016/s0891-5849(03)00110-2
10. Wei H., Frei B., Beckman J.S., Zhang W.J. (2011) Copper chelation by tetrathiomolybdate inhibits lipopolysaccharide-induced inflammatory responses in vivo. Am. J. Physiol. Heart Circ. Physiol., 301(3), H712-H720. DOI: 10.1152/ajpheart.01299.2010
11. Craciun L., Muroy S.E., Saijo K. (2024) Role of copper during microglial inflammation. bioRxiv [Preprint], 10.1101/2024.09.18.613750. 10.1101/2024.09.18.613750. DOI: 10.1101/2024.09.18.613750.DOI
12. Goshi N., Morgan R.K., Lein P.J., Seker E. (2020) A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflammation, 17(1), 155. DOI: 10.1186/s12974-022-02391-4
13. Стельмашук Е.В., Капкаева М.Р., Розанова Н.А., Александрова О.П., Генрихс Е.Е., Обмолов В.В., Новикова С.В., Исаев Н.К. (2022) Влияние индуктора нейровоспаления на компоненты нейроваскулярной единицы головного мозга in vitro. Российский физиологический журнал им. И.М. Сеченова, 108(5), 686-696. DOI: 10.31857/S0869813922050107
Stelmashook E.V., Kapkaeva M.R., Rozanova N.A., Alexandrova O.P., Genrikhs E.E., Obmolov V.V., Novikova S.V. Isaev N.K. (2022) The in vitro effect of the neuroinflammation inducer on brain neurovascular unit components. J. Evol. Biochem. Phys., 58(3), 856-864. DOI: 10.1134/S002209302203019X
14. Stelmashook E.V., Alexandrova O.P., Genrikhs E.E., Novikova S.V., Salmina A.B., Isaev N.K. (2022) Effect of zinc and copper ions on cadmium-induced toxicity in rat cultured cortical neurons. J. Trace Elem. Med. Biol., 73, 27012. DOI: 10.1016/j.jtemb.2022.127012
15. Karve I.P., Taylor J.M., Crack P.J. (2016) The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol., 173(4), 692-702. DOI: 10.1111/bph.13125
16. Genrikhs E.E., Shedenkova M.O., Voronkov D.N., Isaev N.K., Stelmashook E.V. (2024) Activation of microglia and astroglia in unilateral focal traumatic brain injury in rats. Bull. Exp. Biol. Med., 178(2), 196-201. DOI: 10.1007/s10517-025-06306-0
17. Saura J., Angulo E., Ejarque A., Casadó V., Tusell J.M., Moratalla R., Chen J.-F., Schwarzschild M.A., Lluis C., Franco R., Serratosa J. (2005) Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J. Neurochem., 95(4), 919-929. DOI: 10.1111/j.1471-4159.2005.03395.x
18. Saura J. (2007) Microglial cells in astroglial cultures: a cautionary note. J. Neuroinflammation., 4, 26. DOI: 10.1186/1742-2094-4-26
19. Coleman J.W. (2001) Nitric oxide in immunity and inflammation. Int. Immunopharmacol., 1(8), 1397-1406. DOI: 10.1016/s1567-5769(01)00086-8
20. Guzik T.J., Korbut R., Adamek-Guzik T. (2003) Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol., 54(4), 469-487.
21. Quintas C., Pinho D., Pereira C., Saraiva L., Gonçalves J., Queiroz G. (2014) Microglia P2Y6 receptors mediate nitric oxide release and astrocyte apoptosis. J. Neuroinflammation, 11, 141. DOI: 10.1186/s12974-014-0141-3
22. More S., Choi D.-K. (2017) Neuroprotective role of atractylenolide-I in an in vitro and in vivo model of Parkinson’s disease. Nutrients, 9(5), 451. DOI: 10.3390/nu9050451
23. Hwang J.H., Kumar V.R., Kang S.Y., Jung H.W., Park Y.-K. (2018) Effects of flower buds extract of Tussilago farfara on focal cerebral ischemia in rats and inflammatory response in bV2 microglia. Chin. J. Integr. Med., 24(11), 844-852. DOI: 10.1007/s11655-018-2936-4
24. Galea E., Feinstein D.L., Reis D.J. (1992) Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci. USA, 89(22), 10945-10949. DOI: 10.1073/pnas.89.22.10945
25. Hamby M.E., Hewett J.A., Hewett S.J. (2006) TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia, 54(6), 566-577. DOI: 10.1002/glia.20411
26. Moriyama M., Fujitsuka S., Kawabe K., Takano K., Nakamura Y. (2018) Zinc potentiates lipopolysaccharideinduced nitric oxide production in cultured primary rat astrocytes. Neurochem. Res., 43(2), 363-374. DOI: 10.1007/s11064-017-2431-5
27. Kim S., Son Y. (2021) Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int. J. Mol. Sci., 22(16), 8800. DOI: 10.3390/ijms22168800
28. Colasanti M., Persichini T., Venturini G., Polticelli F., Musci G. (2000) Modulation of the nitric oxide pathway by copper in glial cells. Biochem. Biophys. Res. Commun., 275(3), 776-782. DOI: 10.1006/bbrc.2000.3396
29. Zhang W., Yang X., Liu J., Pan Y., Zhang M., Chen L. (2022) Senescent phenotype of astrocytes leads to activation of BV2 microglia and N2a neuronal cells death. Molecules, 27(18), 5925. DOI: 10.3390/molecules27185925
30. Canedo T., Portugal C.C., Socodato R., Almeida T.O., Terceiro A.F., Bravo J., Silva A.I., Magalhães J.D., Guerra-Gomes S., Oliveira J.F., Sousa N., Magalhães A., Relvas J.B., Summavielle T. (2021) Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine. Neuropsychopharmacology, 46(13), 2358-2370. DOI: 10.1038/s41386-021-01139-7
31. Silva A.I., Socodato R., Pinto C., Terceiro A.F., Canedo T., Relvas J.B., Saraiva M., Summavielle T. (2024) IL-10 and Cdc42 modulate astrocyte-mediated microglia activation in methamphetamine-induced neuroinflammation. Glia, 72(8), 1501-1517. DOI: 10.1002/glia.24542
32. Zanier E.R., Fumagalli S., Perego C., Pischiutta F., de Simoni M.G. (2015) Shape descriptors of the “never resting” microglia in three different acute brain injury models in mice. Intensive Care Med. Exp., 3(1), 39. DOI: 10.1186/s40635-015-0039-0
33. Hanisch U.-K., Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci., 10(11), 1387-1394.
Выпуск
Другие статьи выпуска
Использование in silico подходов для оценки потенциальных нежелательных реакций новых фармацевтических субстанций позволяет уменьшить риски, а также финансовые и временные затраты, связанные с разработкой лекарственных средств. С помощью разработанного нами ранее метода выявления химических мотивов, ассоциированных с определёнными типами нежелательной биологической активности, мы оценили “off-target” токсичность клинически исследуемых фармацевтических субстанций, чтобы оценить потенциальные риски их дальнейшего исследования и использования в клинической практике. Для этого созданы структурные фрагменты, высокоспецифичные для ингибиторов рецептора эпидермального фактора роста и дипептидилпептидазы 4 — двух молекулярных мишеней, ассоциированных с широким спектром нежелательных реакций. Проведён поиск соединений, содержащих созданные фрагменты, среди 12070 записей базы данных PubChem, содержащих информацию о проведении клинических испытаний. Показано, что пять соединений, исследуемых в фазах I и II, могут обладать неблагоприятным соотношением “польза-риск”, возникающим из-за потенциального ингибирования одного из двух анализируемых ферментов. Применение подобных аналитических стратегий на ранних доклинических этапах разработки может значительно снизить совокупные финансовые и временные затраты, способствуя ускоренному выводу на рынок более безопасных и доступных лекарственных средств.
Сепсис-ассоциированная энцефалопатия (САЭ) представляет собой острую дисфункцию головного мозга, которая возникает при отсутствии первичного очага инфекции в центральной нервной системе. Целью нашей работы было проведение пилотного нецелевого метаболомного исследования плазмы крови пациентов с САЭ для выявления метаболических изменений, потенциально связанных с патологическим состоянием, и формирования гипотез для дальнейшего изучения патогенеза, поиска перспективных биомаркеров и оценки тяжести состояния септического пациента. Метаболомное профилирование осуществлялось методом ВЭЖХ-МС-ВР с последующим статистическим анализом полученных данных. В результате слепого рандомизированного контролируемого клинического исследования выявлено существенное различие в метаболических профилях основной и контрольной групп. Функциональный анализ позволил обнаружить метаболические пути, наиболее затронутые патологическими процессами у пациентов с САЭ: метаболизм ацилкарнитинов, лизофосфатидилхолинов, таурина, биосинтез фолата и метаболизм лекарственных препаратов — субстрата цитохрома Р450. У больных с САЭ с нарушением сознания в виде делирия и комы отмечено снижение уровня длинноцепочечных ацилкарнитинов и содержания лизофосфатидилхолинов. Метаболомные профили пациентов с САЭ значимо различались в группе умерших и выживших пациентов: концентрации серосодержащих аминокислот в группе умерших были значительно ниже, чем в группе выживших. В нашем исследовании установлены 64 кандидата в биомаркеры, которые потенциально могут быть использованы для прогнозирования исходов сепсиса, что требует дальнейшего изучения с использованием расширенной и независимой когорты пациентов.
Пролекарственные бифармакофорные конъюгаты на основе пиридоксина и наиболее мощного из всех известных НПВС анальгетика кеторолака in vivo проявляют сопоставимую с кеторолаком анальгетическую активность, но при этом обладают значительно более высокой безопасностью и пролонгированностью действия. В настоящей работе in vitro исследованы антиоксидантные и протекторные свойства двух пролекарственных бифармакофорных конъюгатов на основе пиридоксина и кеторолака, их ингибирующая активность в отношении циклооксигеназы (ЦОГ), а также внутриклеточная проницаемость на модели клеток кишечника линии Сасо-2. Показано, что данные соединения ингибируют ЦОГ-1 и ЦОГ-2 на уровне кеторолака со значениями IC50 в интервале от 12,0 мкМ до 34,7 мкМ. Они оказывают выраженное протекторное действие в условиях теплового и химического воздействия мочевины и лимонной кислоты в отношении альбумина и могут проникать в клетки посредством пассивной диффузии.
Фактор некроза опухоли-α (TNFα) — ключевой провоспалительный цитокин, повышение уровня которого наблюдается при воспалительных заболеваниях верхних дыхательных путей. В работе исследовано дозо- и времязависимое влияние TNFα (1–100 нг/мл, 6–48 ч) на линию клеток RPMI 2650 — модели назального эпителия. Кратковременное воздействие (6 ч) вызывало активацию NF-κB и повышение уровня белков межклеточных контактов E-кадгерина и ZO-1 без существенного влияния на жизнеспособность. Продолжительная экспозиция (24–48 ч) приводила к увеличению уровня про-IL-1β, активации апоптоза и снижению жизнеспособности клеток. При этом отмечалось снижение уровня белков межклеточных контактов. Таким образом, при кратковременном воздействии TNFα может оказывать защитное действие, повышая плотность межклеточных контактов, а при увеличении длительности экспозиции он запускает процессы апоптоза и снижает плотность межклеточных контактов, что может способствовать повышению проницаемости клеточного слоя.
Мультиформная глиобластома (ГБМ) — наиболее агрессивная первичная опухоль головного мозга, характеризующаяся крайне неблагоприятным прогнозом. Трудности в диагностике и мониторинге данного заболевания создают необходимость поиска минимально инвазивных подходов, среди которых перспективным направлением считается жидкостная биопсия. Данный обзор посвящен анализу результатов современных исследований, направленных на поиск циркулирующих белковых биомаркеров ГБМ в плазме и сыворотке крови. В качестве биомаркеров рассматриваются свободно циркулирующие белки плазмы крови и белки, находящиеся в составе внеклеточных везикул (ВнВ). В обзоре обобщены результаты работ, использующих для поиска белковых биомаркеров как иммунохимические методы, так и масс-спектрометрические подходы, а также представлен перечень выявленных потенциальных диагностических и прогностических биомаркеров. Анализ представленных в литературе работ показывает, что протеомный анализ, сосредоточенный на фракции ВнВ плазмы крови, существенно расширяет возможности поиска биомаркеров для неинвазивной диагностики и мониторинга ГБМ.
Эпидемиологические исследования показывают, что во всём мире, в том числе в РФ, наблюдается устойчивый рост числа пациентов с когнитивными нарушениями, связанными с нейродегенеративными заболеваниями и различными аффективными расстройствами. В связи с этим существует запрос на разработку более действенных терапевтических подходов к их коррекции. Установлено, что регулярная физическая нагрузка способствует улучшению когнитивных функций и подавляет симптомы депрессии. Работающие мышцы секретируют биологически активные вещества — миокины, регулирующие восстановление самих мышц, а также регулирующие функции внутренних органов, желёз внутренней секреции, иммунной системы и мозга. Результатом является скоординированный ответ органов и систем, направленный на восстановление функциональной активности организма после физической нагрузки. В частности, улучшается память и способность к обучению. Пациенты с когнитивными нарушениями или депрессией часто не способны вовлечься в регулярную физическую активность из-за физических ограничений или ослабления мотивации. В связи с этим фармацевтические препараты, имитирующие эффекты мышечной активности, являются перспективной терапевтической опцией. Одним из направлений может стать создание препаратов на основе миокина иризина, который вырабатывается во время физической нагрузки и оказывает целый ряд благотворных эффектов на когнитивные функции и настроение. В этом обзоре представлены данные по влиянию физической нагрузки на когнитивные функции в норме и при патологии, описано физиологическое действие иризина, представлены предполагаемые механизмы действия иризина на когнитивные функции и симптомы депрессии.
Статистика статьи
Статистика просмотров за 2026 год.
Издательство
- Издательство
- ИБМХ
- Регион
- Россия, Москва
- Почтовый адрес
- 119121, Россия, г. Москва, ул. Погодинская, д. 10, стр.8
- Юр. адрес
- 119121, Россия, г. Москва, ул. Погодинская, д. 10, стр.8
- ФИО
- Пономаренко Елена Александровна (Директор)
- E-mail адрес
- dir@ibmc.msk.ru
- Контактный телефон
- +7 (499) 2466980